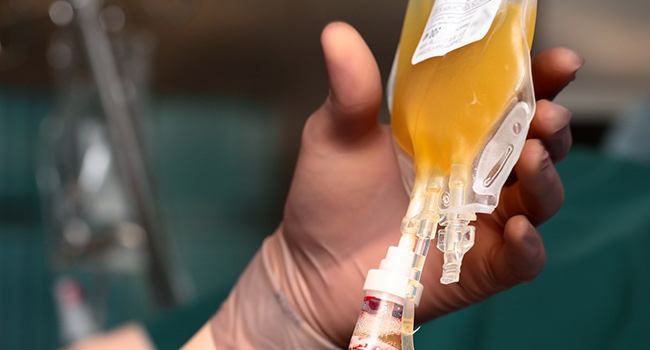
[ad_1]
Father Robert Pace, Fort Worth, TX.
John Burk, MD, pulmonologist, Texas Health Harris Methodist Hospital, Fort Worth.
Michael Joyner, MD, professor of anesthesiology, principal investigator, Convalescent Plasma Expanded Access Program, Mayo Clinic, Rochester, MN.
Elliott Bennett-Guerrero, MD, medical director of perioperative quality and patient safety; professor, Renaissance School of Medicine, Stony Brook University, NY.
Julia Sabia Motley, 57, Merrick, NY.
Mayo Clinic: “Mayo Clinic named national site for Convalescent Plasma Expanded Access Program.”
FDA: “Recommendations for Investigational COVID-19 Convalescent Plasma.”
The Journal of the American Medical Association: “Treatment of 5 Critically Ill Patients With COVID-19 With Convalescent Plasma.”
Press Medicine: “Treatment of Argentine hemorrhagic fever with convalescent’s plasma. 4433 cases.”
Proceedings of the National Academy of Sciences of the United States of America: “Effectiveness of convalescent plasma therapy in severe COVID-19 patients.”
News release, FDA: “Coronavirus (COVID-19) Update: Daily Roundup, March 24, 2020.”
News release, Stony Brook Medicine: “Volunteers Needed to Donate Blood Plasma to Help Hospitalized Patients with COVID-19,” April 9, 2020.
News release, FDA: “Coronavirus (COVID-19) Update: FDA Coordinates National Effort to develop Blood-Related Therapies for COVID-19,” April 3, 2020.
News release, Takeda: ”Global Plasma Leaders Collaborate to Accelerate Development of Potential COVID-19 Hyperimmune Therapy,” April 6, 2020.
Open Forum Infectious Diseases: “High-Dose Intravenous Immuoglobulin as a Therapeutic Option for Deteriorating Patients With Coronavirus Disease 2019.”