[ad_1]
What your doctor is reading on Medscape.com:
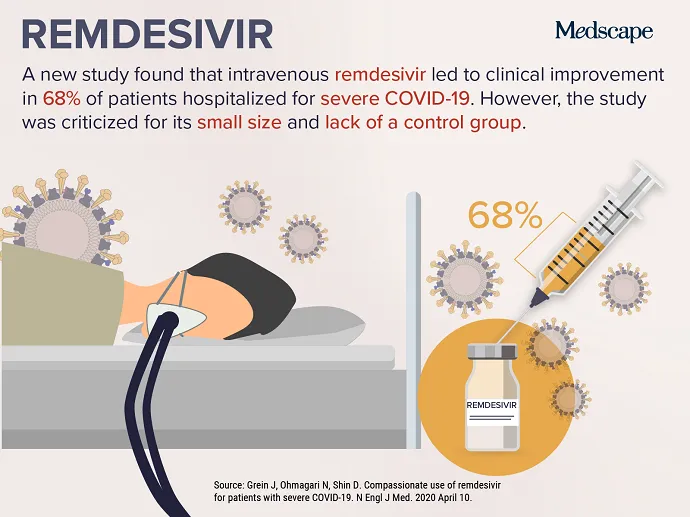
APRIL 24, 2020 — As the COVID-19 pandemic continues, efforts to identify a safe and effective therapy have intensified, leading to this week’s top trending clinical topic. New data on the investigational antiviral drug remdesivir were published April 10 in the New England Journal of Medicine . More than two thirds of severely ill patients with COVID-19 who were given remdesivir for “compassionate use” improved after receiving the medication. Of the 53 patients included in the study, 22 were in the United States, 22 were in Europe or Canada, and 9 were in Japan. Patients received a 10-day course of remdesivir, consisting of 200 mg administered intravenously on day 1, followed by 100 mg daily for the remaining 9 days of treatment.
Although some were encouraged by the results, others warned that limitations in the study make interpreting the data challenging. Josh Farkas, MD, an intensivist from Vermont, provided 11 reasons why the study “reveals nothing” and called the paper “hot garbage” on Twitter. Other physicians lamented the erosion of the peer-review process during the COVID-19 pandemic. The lack of a control group and a small patient sample size have been frequently cited as significant concerns.
In a letter to Gilead, the company behind remdesivir, John Mandrola, MD, calls for blinding and a placebo arm to be added to future trials. Although he applauds the efforts made thus far, Mandrola reiterates that these additions would make the findings more reliable, as “they would be free from the potential biases of clinicians—who, out of the desire to have an available treatment, may consciously or unconsciously make or delay decisions to reduce oxygen and avoid mechanical ventilation in patients in the remdesivir arm.”
At this point, an expert panel of the Infectious Diseases Society of America (IDSA) says evidence is insufficient to recommend any potential pharmacologic therapies for routine use in patients with COVID-19. The effort to validate a treatment approach is massive and global. The World Health Organization has identified a list of “promising candidates” for COVID-19 treatment, including remdesivir, lopinavir-ritonavir, immunotherapies, and convalescent sera. Around 60 trials involving these therapies have been planned, are recruiting, or have already begun. As the results of those trials become available, the drugs studied will receive immense attention, just as remdesivir did this week.